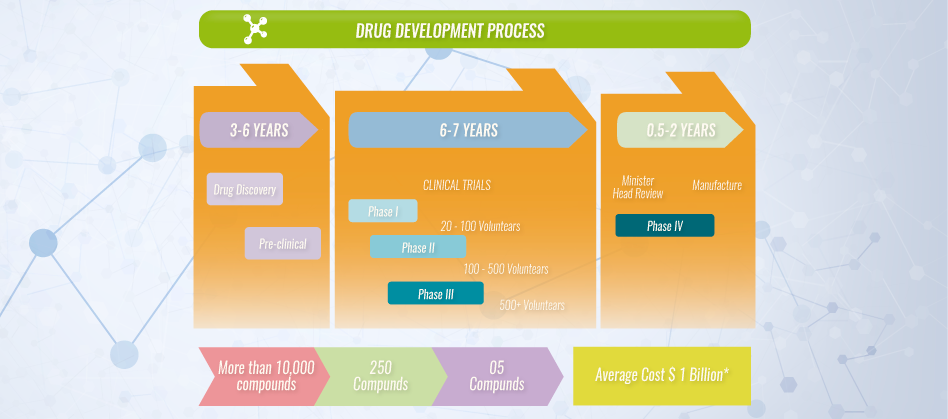
Researchers look for new insights to the causes of disease at a cellular, molecular, proteomic and genetic level so that they can find new targets to direct treatment to cure disease. Once a target is found, the right molecule or compound that has a possible beneficial effect against the target must be found. Many potential candidates or new ways to target the product to a specific site are tested. At this stage there may be thousands of potential compounds for development as a medical treatment, however only a few are determined to be the most promising and are further studied. It takes an average of 10 to 15 years to develop a new medicine from discovery to treating the patient population.
Pharmaceutical and biotech companies invest on the compounds that may lead to safe and effective treatments for patients. The average cost of research and development for each new medicine that reaches the market has been estimated to go from $800 million to $1 billion USD. These figures take into account all the testing for compounds that fail and the contributions of government, academic and industry research. Approximately one of every 5,000 to10,000 compounds that go into research and development receives regulatory authority approval.
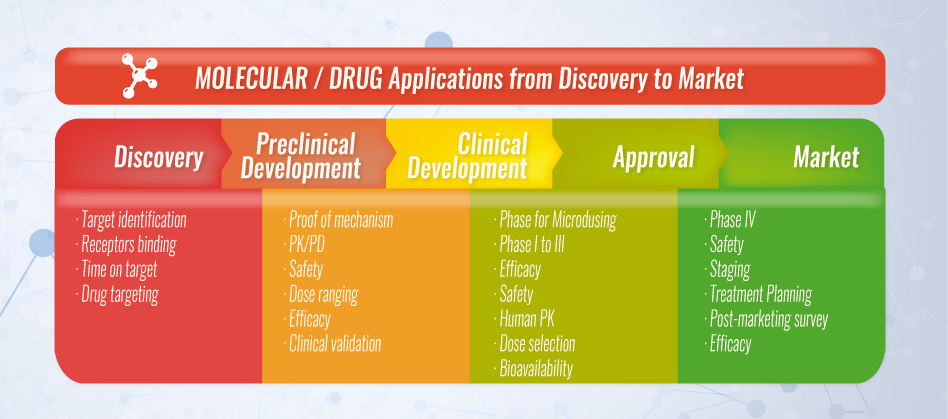
The process can be summarized as follows:
Before testing a new compound in people, researchers have to determine its toxicity. The two types of preclinical research are in vitro and in vivo. Preclinical testing in lab animals has to comply with Good Laboratory Practice (GLP) and must provide sufficient and detailed information on dosing and toxicity levels. The findings are then reviewed and it is determined if the compound will be tested in humans. Regulatory authorities are very strict and require thorough testing prior to approving tests in human subjects.
During this stage, researchers may optimize the compound to make it safer and more efficient and how to make large enough quantities of the compound for clinical trials has to be determined. If approved for use in the general population greater scaling of production is needed.
Phase 1 studies are designed for initial human testing in a small group (20-100) of healthy volunteers or people with the disease/condition. The main goal of a Phase 1 trial is to discover if a drug is safe for use in humans. Pharmacokinetics and pharmacodynamics are studied during this time to help researchers determine what the safe dosing range is and if it should move on to the next stage of development.
The percentage of drugs that move on to the next phase is 70%.
Test in a small group of patients Phase 2 trials researchers evaluate the drug’s effectiveness in a group of 100-500 patients with the disease/condition under study, and evaluate adverse events and risks associated with short-term use of the drug. Optimal dose strength and schedules for using the drug are also studied.
The percentage of drugs that move on to the next phase is 33%.
Test in a large group of patients to show safety and efficacy
In Phase 3 trials the drug is studied in a larger population (about 300-5,000) of patients to test the drug for safety and efficacy, in order to determine the risk-benefit relationship of the drug. It also provides most of the elements for labeling and prescribing instructions to ensure proper use.
The percentage of drugs that move to the next phase is 25-30%
Once all three clinical trial phases are completed the, if the finding demonstrate the drug is safe and effective, the sponsor company can then apply for regulatory authority review of all the information generated, including manufacturing and labeling information - which is usually tens of thousands of pages – in order to obtain a marketing approval. Regulatory authority experts review the information presented and if they find that the information demonstrates the safety and efficacy of the study drug it is approved for use by the general public. If not found to be sufficient they might require additional information or studies before approval is given, or they might deny approval completely.
Research on a new drug continues even after approval. Since a larger number of patients will begin to use the drug, the sponsor companies must monitor its use and submit periodic safety reports. In addition, sometimes it might be required to conduct additional evaluations on an approved drug with a “Phase 4” study. These trials are usually performed on several thousand volunteers to evaluate long-term safety, efficacy or to determine its effects on a specific subgroup of patients.